Abstract
Background
Rivaroxaban, a direct oral anticoagulant (DOAC), has been shown to be an efficacious and safe agent for treatment and secondary prevention of thromboembolism (TE). Currently, there is limited evidence available regarding its use in individuals weighing > 120 kg. Given the increasing prevalence of obesity in the general population, and that elevated weight itself is a risk factor for TE, this lack of data is a substantial issue. Theoretically, the greater volume of distribution in obesity may decrease DOAC peak drug concentration and half-life, potentially resulting in sub therapeutic anticoagulation. In fact, current ISTH guidelines recommend against empiric use of DOACs in patients over 120 kg, with the caveat that their use may be considered if drug specific peak and trough levels fall within the expected range (Martin et al. J Thromb Haemost. 2016). Additional data is required to guide the clinician regarding DOAC use for the management of TE in this growing demographic.
Objectives
Our primary objective was to assess peak rivaroxaban plasma levels (PRPL) in patients weighing > 120 kg on the standard 20 mg daily dose for treatment or secondary prevention of VTE and to compare these values to the expected range derived from pharmacokinetic studies in non-obese patients. The secondary objective was to assess for TE complications in this cohort.
Methods
Electronic patient records were retrospectively reviewed at a thrombosis clinic at a single center from January 1, 2014 to May 31, 2017. Patients of age > 18, on rivaroxaban for treatment or secondary prevention of VTE, of weight > 120kg, and with at least one PRPL, measured 2-4 hours post drug ingestion at steady state, were included in the study. Patients with decreased renal function (creatinine clearance < 30mL/min), liver dysfunction (Child-Pugh score B or C), on concomitant strong P-glycoprotein or CYP 3A4 inducers or inhibitors, or with an additional indication for anticoagulation were excluded. Reference ranges for PRPL were extracted from pharmacokinetic studies (Buller et al. Blood. 2008). PRPL were measured using an anti-Xa assay calibrated to rivaroxaban (BIOPHENTM DiXal, HYPHEN BioMed).
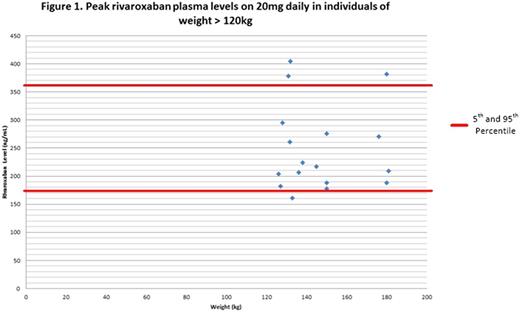
Results
There were 17 unique patients, each with a PRPL, weighing > 120kg, on rivaroxaban for treatment or secondary prevention of TE, over our study period. Six were female. The mean weight was 146.75 (SD +/- 20.19) kg. The mean calculated creatinine clearance (Cockcroft-Gault) was 208.53 (SD +/- 57.36) mL/min. The mean length of follow up from measurement of PRPL was 312.29 (SD +/- 265.47) days.
The mean PRPL was 248.51 (5th to 95th percentile 174.6-380.4) ng/mL compared to the reference of 5th to 95th PRPL percentile of 175-360 ng/mL in non obese patients. Only 1 PRPL was found to be below the expected range at 161ng/mL (see figure 1). There were no recurrent TE events in any of the patients during the study.
Conclusions
We found that the majority of individuals weighing > 120 kg on standard dose rivaroxaban for treatment and secondary prevention of TE had a PRPL within the expected range, with only one value out of the seventeen falling below that range. There were no recurrent TE events in any of the patients. It is possible that the increased volume of distribution in obesity played a lesser role in decreasing PRPL given the lower lipophilicity of rivaroxaban compared to apixaban and dabigatran, although similar to edoxaban (Remko. Journal of Molecular Structure: THEOCHEM. 2009, Remko et al. Molecules. 2016). Despite the small sample size and lack of other pharmacokinetic values, this preliminary data is clinically reassuring that the use of rivaroxaban in patients weighing > 120kg seems to be safe. Further studies are required to fully elucidate the efficacy and safety of DOACs in the treatment of TE in morbidly obese patients.
Sholzberg: Shire: Honoraria, Research Funding; CSL Behring: Honoraria, Research Funding; Octapharma: Honoraria, Research Funding; NovoNordisk: Honoraria. Yeo: Bayer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal